How weight-loss jabs could stop cancer coming back: Scientists reveal breakthrough… and tell why thousands of women with breast cancer may soon be told they don’t need ANY treatment
Roughly 56,000 British women each year are given the terrifying news that they have breast cancer.
It’s a life-changing diagnosis not least because, almost always, it is followed by invasive biopsies, surgery, radiotherapy, chemotherapy and hormone treatments that, in younger women, induce the menopause.
But could all that be set to change? Indeed, could many thousands of women told they have the disease not need a jot of treatment whatsoever?
The provocative answer, according to a growing number of the world’s leading specialists, is yes. The results of a major trial, announced last week, showed it was perfectly safe – perhaps even a better option – to simply ‘watch and wait’ with certain breast cancers, known as ductal carcinoma in situ, or DCIS.
No surgery, no medication – just a check-up twice a year. DCIS are tiny tumours, confined to the milk ducts in the breasts, which usually cause no symptoms and cannot be felt.
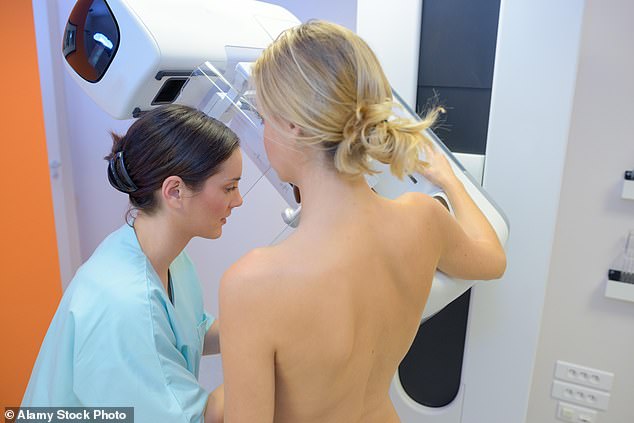
They account for about a quarter of all breast cancer diagnoses and most are discovered only during routine mammograms (breast X-rays).

Experts have long wondered whether some tumours would, if left alone, never grow or spread
Indeed, before the introduction of breast screening, DCIS was rarely seen.
Experts have long wondered whether some, if not many of these tumours would, if left alone, never grow or spread.
But, in the absence of a definitive answer, the standard approach is to err on the side of caution and treat them all.
However, the new trial data, unveiled at the San Antonio Breast Cancer Symposium last week, is one of the strongest signs yet that this may be unnecessary for low-risk DCIS.
And the experts I spoke to suggested within the next five years, if further research backs it up, we could see a radically different approach to treating the 14,000 annual cases of DCIS in the UK. Perhaps even more startlingly, some now suggest we should stop referring to DCIS as a type of cancer at all.
‘We’ve always treated DCIS as a cancer, but these results are casting it in a very different light,’ said Professor Eun-Sil Shelley Hwang, an expert in radiology at Duke University in North Carolina, who was involved in the study.
Breast cancer specialist and author Dr Liz O’Riordan added: ‘Radiotherapy, surgery, hormone treatment tamoxifen all have side effects. Women with breast cancer who have to undergo these treatments often struggle.
‘It’s promising to see that in another four, five years, we may actually be able to say to some, actually, you don’t need to have surgery, we can just monitor you, it’s safe to do that.’
The trial compared outcomes of women with DCIS who were given the standard therapies with those simply monitored using scans and physical exams every six months.

The standard approach has been to err on the side of caution and treat all tumours
Volunteers could elect to have surgery or hormone treatment at any time and it was given if the tumour showed signs of invasive progression. After two years, those who weren’t treated were at no higher risk of developing a more invasive form of the cancer than those who were.
The ‘watch and wait’ group also reported a better quality of life and far fewer issues such as post-surgical arm swelling – a common complication after breast tumour ops – or breast pain. Consultant obstetrician Susan Bewley, Emeritus Professor of Obstetric and Women’s Health at King’s College London, welcomed the study’s findings – and suggested it was time to stop calling DCIS cancer.
‘When breast screening was introduced [in 1988] we began picking up DCIS. However, there is good evidence that this didn’t improve breast cancer survival rates,’ she said.
‘It’s quite possible that invasive procedures like biopsies, which involve sticking a needle into the tumour to take a tissue sample, and other treatments, actually accelerates the growth of these growths. But if we left them alone, they would be harmless. In which case we’ve given a cancer diagnosis, with all the worry that comes with, that’s unnecessary.
‘The saying “let sleeping dogs lie” springs to mind, as I believe that’s what we need to do with many cases of DCIS. And if they do wake up, we have brilliant treatments that can offer a cure.
‘It’s just a shame we didn’t have this research four decades ago.’
Glasgow-based breast cancer surgeon Dr Julie Doughty added: ‘We aren’t there yet, but in time there may be certain women with low-grade DCIS that doctors could have a conversation with about taking a watch-and-wait approach.’
But, of course, it wasn’t all about DCIS at the world-renowned conference. Weight-loss jabs to stop the disease in its tracks, powerful radiotherapy that spares women half of their mandatory appointments, and a pill so potent it could significantly slash the global death toll among women with genetic breast cancer were just some of the other breakthroughs reported.
Read on to learn more about the exciting discoveries…
New drug hope for incurable cases
Around two-thirds of breast cancer patients in the UK have a form of the disease called ER positive HER-2 negative breast cancer. Of these, up to half with an incurable form of the disease can develop what is known as an ESR1 mutation during hormone treatment, meaning their cancer has become resistant to treatment. And for these women, the outlook is incredibly bleak.
But a new trial combining a daily pill called imlunestrant with another drug abemaciclib – commonly prescribed drug for this form of the disease – led to a 43 per cent reduction in death in this group.
Taken on its own, imlunestrant also reduced the risk of death by 38 per cent. The drug works by preventing oestrogen – the female hormone that fuels many breast cancers – from attaching to tumour cells, so they stop growing.
‘Imlunestrant is revolutionary,’ said Professor Komal Jhaveri, an oncologist and early drug development specialist at the Memorial Sloan Kettering Cancer Centre in New York, who also presented the research.
The Mail on Sunday understands imlunestrant’s manufacturer, Eli Lilly, has now submitted an approval application with UK drugs regulator the Medicines and Healthcare products Regulatory Agency. It is tipped for approval as soon as next year.
‘This is the first time we’re seeing such positive results in this patient group,’ Dr Jhaveri said.
‘I truly think it’s a great option. We’re all really excited.’
But this trial wasn’t the only good news from San Antonio for women with this aggressive form of the disease.
Latest figures from separate British-led research into a similar daily pill known as camizestrant posted equally promising results.
The AstraZeneca-backed tablet in combination with another common breast cancer medication, ribociclib, bought patients an average of eight extra months – but some had survived for more than five years and were still well.
Dr Richard Baird, the Cambridge-based oncologist who was lead investigator of the trial, said: ‘These results are really encouraging. It also proves there is important clinical research happening in the NHS.
Ozempic can stop cancer returning
Breakthrough weight-loss jabs – such as Ozempic, Mounjaro and Wegovy – are incredibly effective at suppressing appetite.
But hopes have now been raised that the jabs could also help protect against breast cancer returning.
One study of more than 1,000 patients by the University of Texas – the largest to date on the topic – found obese patients who took the drugs for just over a year after finishing treatment on average had a ‘significantly improved’ chance of living longer.
However, surprisingly, they also found patients taking hormone drugs such as tamoxifen – given to many women to stop breast cancer recurrence – gained weight, despite using the jabs.
Experts admit they are unsure why this happened but suggest it may be because hormone therapy typically causes patients to gain weight, meaning the jabs aren’t able to work effectively.
For these patients, ‘we may need to go up to a higher dose’, said Professor Neil Iyengar, an oncologist at the Memorial Sloan Kettering Cancer Centre, New York.
In another trial, launched last month, obese patients undergoing treatment for HR+/HER2- breast cancer – the most common form of the disease – will be given weight-loss jab tirzepatide, also known by brand name Mounjaro, for two years to assess recurrence risk.
Dr Coral Omene, an associate professor of medicine at Rutgers Cancer Institute in New Jersey and lead researcher, said: ‘The evidence overwhelmingly suggests these drugs have a positive effect on preventing not just breast but other cancers. I think this trial will be positive.’
Prof Iyengar added: ‘We’ve known for at least two decades now that obesity is a major risk factor for recurrence.
But the problem with most weight-loss medications previously is they were too toxic and just not as effective.
‘Now we may actually have a drug therapy we can offer patients to lower breast cancer recurrence risk.’
Angelina gene pill cuts risk of death
A daily pill can drastically slash the risk of women dying from one of the most aggressive types of cancer, scientists have discovered.
Experts have known for some time that around five per cent of breast cancers are caused by a faults in a gene called BRCA. These cancers, if they do develop, are notoriously aggressive. Famously, actress Angelina Jolie discovered she carried the BRCA mutation, and underwent a preventative mastectomy in 2013 to reduce her risk of the disease.
The new drug, olaparib, is already offered on the NHS to women with BRCA breast cancers, but the new research has proven just how effective it is.

Actress Angelina Jolie discovered she carried the BRCA mutation and had a preventative mastectomy in 2013 to reduce her risk of the disease
After six years, women with high-risk, early-stage breast cancer who had olaparib added to their treatment, saw their risk of death reduced by 28 per cent. It also cut the likelihood of their cancer returning by 35 per cent.
Professor Andrew Tutt, an oncology expert at The Institute of Cancer Research in London who was involved in the trial, said olaparib ‘exploits the weaknesses’ of BRCA cancers, halting their growth.
He added that the trial was further proof of how important genetic testing was in women diagnosed with early-stage breast cancer – to identify who should benefit as quickly as possible.
‘These are really good results for our patients,’ said Dr Judy Garber, head of cancer genetics and prevention at the Dana-Farber Cancer Institute in Boston.
We should avoid radiotherapy
Thousands of breast cancer patients could see their radiotherapy appointments halved, thanks to a new targeted approach being studied by international researchers.
The method involves giving patients a higher-than-usual dose of radiation once a day for 15 days – rather than 30 days of the standard dose. Although the trial has been running for just over a year, to date, no major side effects have been reported in women given the bigger doses.
Radiotherapy is typically given after breast cancer surgery to stop the disease returning. Previous smaller studies have also suggested fewer, high-dose sessions are equally effective in stopping recurrence.
‘A radiotherapy session itself only lasts five minutes, but appointments often take around an hour,’ said Dr Courtney Pisano, lead researcher at University Hospitals Cleveland.
‘It’s a huge burden and often leads to women having to take more time off work, spending more time travelling and makes childcare challenging.
‘Our trial is a more convenient way to deliver radiation.’
Elsewhere at the conference, Professor Ian Kunkler, a clinical oncologist at the University of Edinburgh, also suggested some women with intermediate risk breast cancer may not need radiotherapy at all.
In a ten-year trial, scientists found that, in this group, having radiotherapy after a mastectomy made ‘no difference in overall survival’ after almost a decade.
‘Often doing less is actually better,’ the consultant clinical oncologist at the University of Edinburgh told this newspaper.
‘This is sometimes difficult to come to terms with, because with breast cancer there’s a tendency to want to add more treatment rather than less.
‘With the exception of certain breast cancers, such as triple negative, we should be avoiding radiation.’